Plasmid DNA preparation is a basic but very important step in scientific research. For years, Synbio Technologies has provided comprehensive plasmid DNA preparation services to our customers from research institutes to both biotechnology and biopharmaceutical industries.
Synbio Technologies conducts comprehensive quality control of our plasmid products to provide you with aseptic plasmids with no RNA pollution or genome pollution. All plasmids we manufacture are free of animal-derived materials and can contain low levels of endotoxin (< 100EU/mg, <30EU/mg, <5EU/mg, on request). According to our customers’ application needs, standard plasmid DNA preparation services are divided into two levels, the research level and the transfection level. The plasmid DNA preparation process is designed to meet diverse downstream applications such as transfection, antibody preparation, vaccine, and gene-therapy research.
Competitive Advantages
- Express and Guaranteed Plasmid Preparation Services: USA based manufacturing allows high quality products and fast delivery.
- Highly Customized: Microgram-to-gram-scale quantity can meet various customers’ needs.
- Low Endotoxin Level: Animal-derived materials free, <100EU/mg, on request.
- Strict Quality Control: ISO9001 & ISO13485 quality management, complete and detailed manufacturing documentation.
- Intellectual Property (IP) Protection: All our customers’ intellectual project related rights are fully respected and protected.
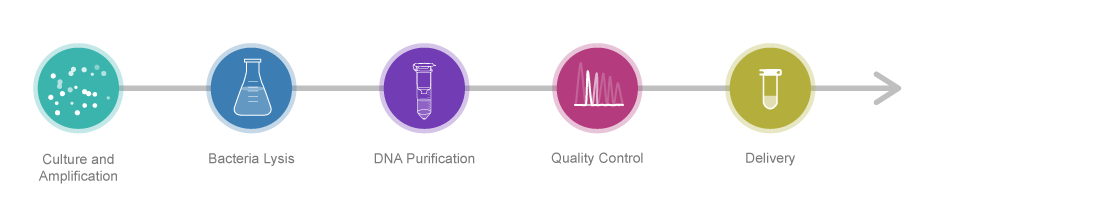
Service Process
| Research Grade | Transfection Grade | ||
|---|---|---|---|
| Endotoxin Level | N / A |
|
|
| Quantity | 2 ug to gram level | 20 ug to gram level | |
| Turnaround time | Starting at 2 business days | Starting at 2 business days | |
| Quality Control |
|
|
|
| Recommended Applications |
|
|
|
| Standard Delivery Package Contains | Prepared lyophilized plasmid DNA (default conc. ~1mg/mL), Certificate of analysis (COA), and QC report | ||
| Standard Plasmid Preparation Package |
Research Grade | Transfection Grade | |
|---|---|---|---|
| Endotoxin Level | NA | <100EU/mg* | |
| Quantity | 0.1 mg to gram level | 1 mg to gram level | |
| Turnaround time | Starting at |
Starting at |
|
| Quality Control Methods | Restriction enzyme analysis |
|
|
| Recommended Applications |
|
|
|
| Standard Delivery Package Contains | Prepared plasmid DNA, Certificate of analysis (COA) and QC report | ||
*Optional endotoxin level: < 100EU/mg, <30EU/mg, <5EU/mg.
Please note:
1. Synbio Technologies provides upstream services of plasmid preparation such as gene synthesis and subcloning into both commercial and custom vectors. This allows us to offer a unique approach to satisfy all our customer’s requests.
2. Additionally, if you wish to provide the plasmid template, you will need to prepare over 100 ng DNA sample (diluted with ddH2O or TE buffer), colonies (fresh) or bacteria stored in glycerol. (Inquiry: quote@synbio-tech.com)
Application Grade Plasmid DNA Preparation
Day 1: Bacteria were transformed by target plasmid DNAs and grown on LB agar plates with proper antibiotics.
Day 2: Bacterial Colonies were selected and cultured in LB medium with proper antibiotics.
Day 3: Mini-prepped plasmid DNAs, followed by restriction enzyme analysis to verify the DNA sizes. In addition, liters of bacteria cultures were inoculated for a large-scale preparation of plasmid DNAs.
Day 4: Bacteria were harvested and the plasmid DNAs were purified.
Quality Test Results(Day 5 – 7):
- Endotoxin Test: Negative results will be not indicated.
- Sterility Test: No bacterial colony is detected after 48 hours culturing in LB medium.
- Restriction enzyme digestion: correct DNA sizes
- Sanger sequencing: correct sequence in specified regions.
Day 8: DNAs were formulated, dispensed, labelled, and delivered.
Synbio Technologies plasmid preparation service focuses on quality. We have strict quality control which can increase quality stability and reduce batch difference to satisfy customers’ high-quality needs.
| QC Items* | Method | Specifications | Default QC Research Grade |
Default QC Transfection Grade |
QC Price |
| Appearance | Visual inspection | Clear, colorless, free from visible particulates. | √ | √ | Free |
| A 260/280 Ratio | UV Absorbance | 1.80 ~ 2.00. | √ | √ | Free |
| Quantity | UV Absorbance | Quantity is ± 5%. | √ | √ | Free |
| Residual RNA | Agarose gel electrophoresis | Not visible | √ | √ | Free |
| Genomic DNA | Agarose gel electrophoresis | Not visible | √ | √ | Free |
| Fragment Size | Restriction Digestion | The size of plasmids fragment is right and free of any contaminated bands. | √ | √ | Free |
| Endotoxin control | TAL assay | < 100EU/mg | x | √ | Free |
| Supercoil content | Agarose gel electrophoresis | Supercoil content >90% | x | √ | Free |
| Bioburden | Streak inoculation | No growth after 48 hours. | x | √ | Free |
| Mycoplasma | Streak inoculation | No mycoplasma is detected in the plasmid products. | x | x | Contact us. |
| Sequencing | Whole plasmids sequencing | Sequencing results are consistent with the confirmed plasmid sequence. | x | x | Contact us. |
Contact us for more QC items.