Nucleic acid probes are usually labeled with a detectable moiety such as radioisotopes, epitopes, fluorophores, or biotin to facilitate the detection of their respective target molecules. With our propriety synthesis platform and professional research team, Synbio Technologies is able to produce high quality nucleic acid probes with batch consistency for both routine and specialized applications. Additionally, we can provide a wide variety of modifications or labeling at the 5′ and 3′ ends or a designated position of the probe according to the specific need of our customers.
Competitive Advantages
- High Purity: Special purification processes ensure efficient removal of small molecule fluorescent residue and purity up to 98%.
- Strict Quality Control: ISO13485:2016 quality control management system ensures real-time monitoring of probe production and batch-to-batch consistency.
- Online Ordering System: Complete online primer/probe synthesis ordering system to streamline the ordering process.
- Diverse Production Scale: Our experienced probe synthesis experts provide nm to uM scale through our optimized production processes.
- Customizable Synthesis: Customized modulations and labeling is available upon request.
Service Specifications
| Modification | Purification | |
|---|---|---|
| TAMRA | 5’FAM-3’TAMRA, 5’HEX-3’TAMRA, 5’TET-3’TAMRA, 5’JOE-3’TAMRA | HPLC/PAGE |
| BHQ1 | 5’FAM-3’BHQ1, 5’HEX-3’BHQ1, 5’VIC-3′BHQ1, 5’NED-3′BHQ1, 5’TAMRA-3′BHQ1, 5’JOE-3’BHQ1 | |
| BHQ2 | 5’Cy5-3’BHQ2, 5’Cy3-3’BHQ2, 5’ROX-3’BHQ2 | |
| MGB | 5’NED-3′ MGB, 5’TAMRA-3′ MGB, 5’ROX-3’MGB, 5’JOE-3’TAMRA, 5’Cy5-3’MGB, 5’Cy3-3’MGB, 5’VIC-3′MGB, 5’HEX-3’MGB, 5’FAM-3’MGB | |
| DABCYL | 5’FAM-3′ DABCYL, 5’HEX-3′DABCYL, 5’JOE-3′DABCYL, 5’ROX-3′DABCYL, 5’TAMRA-3′DABCYL, 5’CY5-3′DABCYL | |
* The above is our suggested purification method. If you need other purification methods, please contact us for a quote.
* The estimated turnaround time for research use is 3-5 days and industrial use is 1 – 2 weeks.
Service Standards for Research or Industrial Use
| Application Level | Research Application | Industrial Application |
|---|---|---|
| Synthetic Specification | nm | um |
| Delivery Time | 3-5 Days | 1-2 Weeks |
| Standard Delivery Project | Sample、COA file、MS or HPLC report | Sample、COA file、MS or HPLC report、Fluorescence full wavelength scan etc |
| Purification | RP-HPLC/PAGE etc | RPHPLC/IE-HPLC/Dual HPLC etc |
| QC | RP-HPLC detection ≥90%, LC-MS detection molecular weight deviation <0.1%, Full wavelength scanning (optional) | RP-HPLC detection ≥95%, IE-HPLC detection ≥95%, LC-MS detection molecular weight deviation <0.1%, full wavelength scanning (optional), others |
| Cross-Contamination | Conventional platform anti-pollution process | Special anti-pollution process |
| Delivery Form | Dry powder | Dry powder |
Case Studies
Purity is one of the main factors affecting the effectiveness of a nucleic acid probe. Synbio Technologies’ advanced and optimized purification techniques can maximize the elimination of remaining raw materials and other impurities from the target probe, as illustrated below.
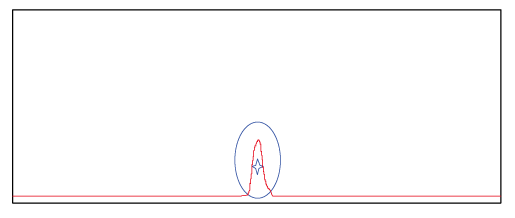
Figure 1. The spectrum shows the peak of probe product purified with the conventional method. ☆ represents the target product and O represents impurities. The spectrum shows that impurities were not successfully separated from the target product.
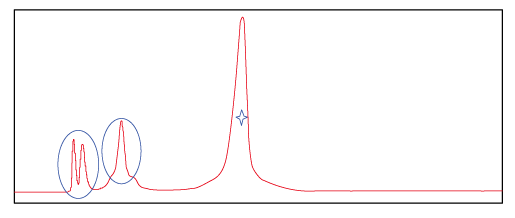
Figure 2.This spectrum shows the peak of probe product purified with Synbio Technologies’ optimized purification method. ☆ represents target product and O represents impurities. The spectrum shows that impurities were further separated from the target product.
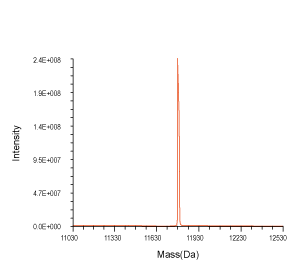
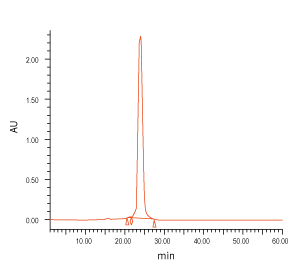
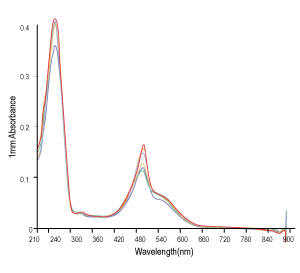
Figure 3. QC analysis of the target product purified with Synbio Technologies’ purification method. The MS (left) analysis shows the mass of the product was comply to its theoretical molecular weight without N± peak; the HPLC (middle) analysis shows the purity of the product was >90%; and the UV (right) shows the absorbance of the product was consistent with its corresponding parameters.

